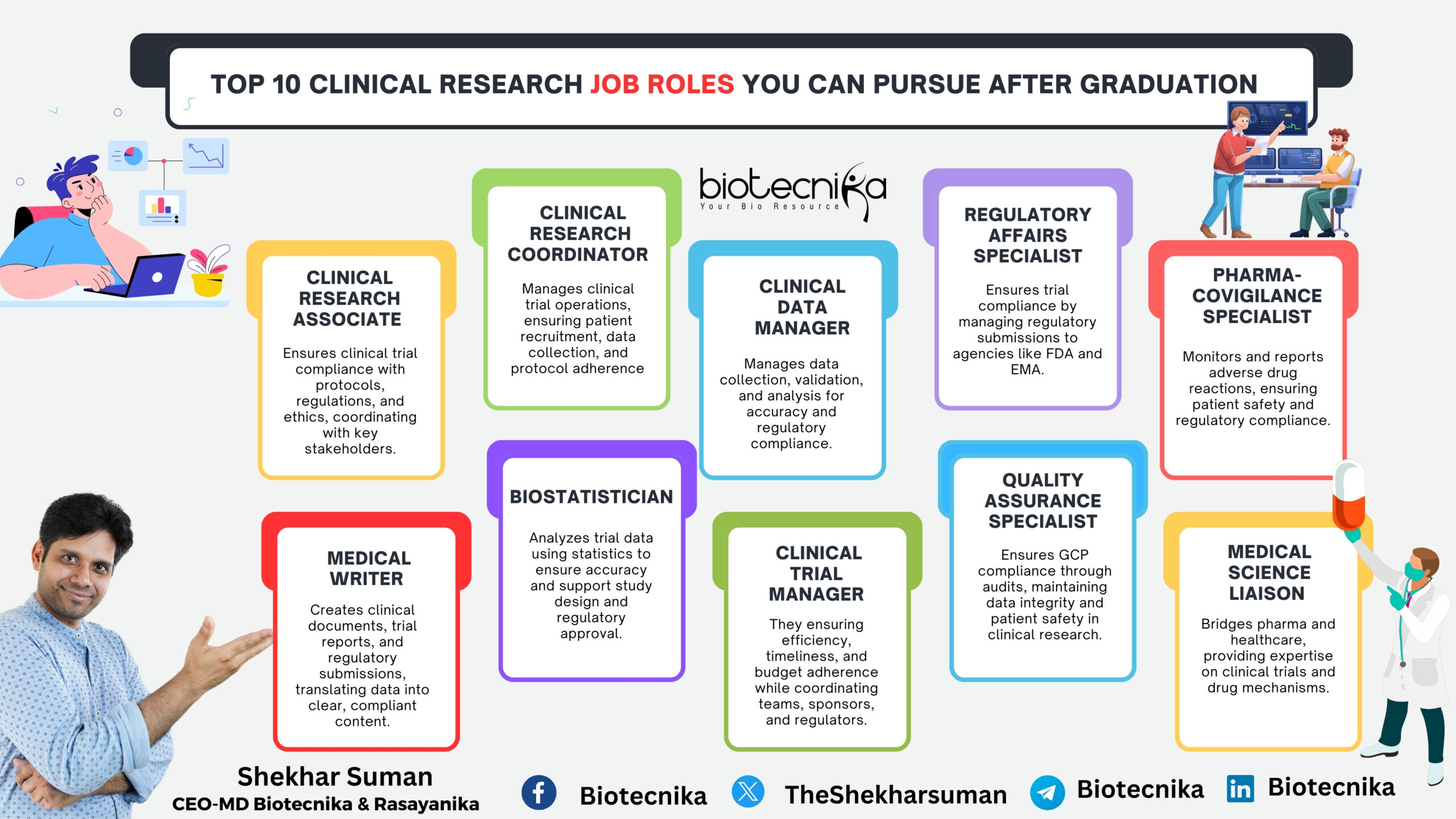
Top 10 Clinical Research Job Roles You Can Pursue After Graduation
Sophia always loved science, but she didn’t want to spend her life pipetting in a lab. In her last year of university, she attended a conference about clinical trials. She realized science could be applied in so many different ways, such as writing medical reports, ensuring drug safety as well as managing entire research projects. The more she explored, the more she realized clinical research was the perfect combination of science, innovation as well as impact.
Clinical research is a rapidly growing field that plays a crucial role in developing new medical treatments, drugs, and therapies. If you have recently graduated with a degree in life sciences, pharmacy, biotechnology, or a related field, there are multiple career paths you can explore in clinical research. If you’re like Sophia, looking for a career beyond traditional lab work, here are 10 exciting roles in clinical research to consider.
Table of Contents
1. Clinical Research Associate
A Clinical Research Associate (CRA) monitors clinical trials to ensure they are conducted ethically and in compliance with regulatory guidelines. They work with pharmaceutical companies, research organizations, and hospitals.
Key Responsibilities:
- Conduct site visits to ensure proper trial procedures.
- Verify research protocols and ensure compliance with Good Clinical Practice (GCP).
- Monitor data collection and report protocol deviations.
- Ensure informed consent is obtained from trial participants.
- Maintain communication between trial sites and sponsors.
Skills Required:
- Attention to detail.
- Strong communication and interpersonal skills.
- Knowledge of regulatory guidelines (ICH-GCP, FDA, EMA).
- Ability to travel frequently for site monitoring.
2. Clinical Data Manager
They play an important role in collecting, organizing as well as analyzing data generated from clinical trials. The clinical data manager ensures the accuracy as well as integrity of trial data, which is essential for regulatory approval.
Key Responsibilities:
- Develop as well as manage the clinical trial database.
- They ensure data validation as well as quality checks.
- Collaborate with statisticians to analyze trial results.
- Work with regulatory agencies to ensure compliance.
- Generate reports and data summaries for submission.
Skills Required:
- Proficient in data management tools such as SAS, R, or SQL.
- Must have strong analytical skills to ensure data accuracy.
- Attention to detail to prevent errors in data collection.
- Teamwork and collaboration with statisticians and clinical teams.
3. Clinical Trial Coordinator
They are responsible for the daily management of clinical trials. They work mainly with principal investigators, patients, and regulatory bodies to ensure that research continues and goes on smoothly.
Key Responsibilities:
- The play main role in organizing and managing patient recruitment for trials.
- They also maintain documents and ensure compliance with the protocol.
- Coordinate with different stakeholders, which includes sponsoring the trial sites.
- Assist in budgeting and financial aspects of clinical trials. They also ensure proper storage and handling of materials.
Skills Required:
- Clinical trial coordinators must have strong multitasking abilities to handle various trial components.
- Candidates must have basic knowledge of regulatory guidelines as well as ethical considerations.
- Organizational skills for maintaining trial documentation.
- Effective communication with healthcare professionals and patients.
4. Regulatory Affairs Specialist
A Regulatory Affairs Specialist ensures that clinical trials comply with regulatory requirements set by agencies like the FDA, EMA, and WHO. This role is essential for bringing new drugs and medical devices to market.
Key Responsibilities:
- Their main role is in preparing and submitting regulatory documents for clinical trials.
- They ensure compliance with local as well as international regulatory bodies.
- Stay updated on changes in clinical research policies.
- Liaise with regulatory bodies for trial approvals.
- Assist in audits and inspections.
Skills Required:
- Strong understanding of global regulatory frameworks.
- Attention to detail to prevent compliance issues.
- Problem-solving abilities for addressing regulatory challenges.
- Excellent documentation and report-writing skills.
5. Clinical Project Manager (CPM)
They oversee the entire phase of a clinical trial, from planning to execution, as well as completion. They make sure that trials meet the deadlines and budget requirements.
Key Responsibilities:
- They focus on developing trial timelines, budgets as well as resource plans.
- Coordinate cross-functional teams involved in clinical trials.
- Identify risks and implement mitigation strategies.
- Ensure compliance with ethical and regulatory standards.
- Communicate trial progress to stakeholders.
Skills Required:
- Strong leadership and decision-making abilities.
- Organizational and time management skills.
- Risk management and problem-solving capabilities.
- Knowledge of clinical trial software like CTMS.
6. Medical Writer
A Medical Writer plays a crucial role in documenting trial findings and preparing for regulatory submissions. This role requires a strong scientific background and the ability to communicate complex information clearly.
Key Responsibilities:
- They should have knowledge of writing clinical research reports, protocols, and informed consent documents.
- Write manuscripts for scientific publications.
- Ensure accuracy and compliance with guidelines.
- Collaborate with researchers and regulatory teams.
Skills Required:
- Strong knowledge of writing and editing abilities.
- Ability to interpret clinical trial data as well as give attention to detail for accurate documentation.
- Familiarity with medical terminology and guidelines like ICH-GCP.
7. Biostatistician
A Biostatistician analyzes clinical trial data to determine the effectiveness and safety of medical treatments. They play an important role in regulatory affairs and decision-making.
Key Responsibilities:
- Design statistical models for clinical trials.
- Analyze and interpret trial data.
- Work with data management teams to ensure accuracy.
- Prepare statistical reports for regulatory submissions.
- Support researchers in study design and methodology.
Skills Required:
- Proficiency in statistical software (SAS, R, SPSS).
- Critical thinking and problem-solving skills.
- Attention to detail for data accuracy.
- Strong mathematical and analytical abilities.
8. Clinical Safety Officer
The clinical safety officer monitors the patients who participate in clinical trials. They assess adverse events and ensure compliance with safety regulations.
Key Responsibilities:
- Ensure, identify, and report adverse drug reactions.
- Follow safety protocols as well as conduct risk assessments for new therapies.
- Maintain pharmacovigilance records as well as collaborate with regulatory teams for safety reporting.
Skills Required:
- Knowledge of pharmacovigilance and drug safety.
- Attention to detail for identifying safety risks.
- Ability to analyze and interpret safety data.
- Strong ethical and compliance awareness.
9. Quality Assurance (QA) Specialist
A Quality Assurance Specialist ensures clinical trials adhere to regulatory and quality standards. This role is vital for maintaining trial integrity.
Key Responsibilities:
- Conduct internal audits of trial procedures.
- Ensure compliance with GCP, FDA, and EMA standards.
- Train staff on quality guidelines.
- Investigate and report quality deviations.
- Maintain detailed audit reports.
Skills Required:
- Understanding of clinical quality management systems (QMS).
- Give attention to detail for quality checks.
- Strong analytical and reporting skills.
- Ability to enforce compliance standards.
10. Clinical Research Scientist
They play a crucial role in designing and implementing innovative clinical trials to discover new drugs, treatments, and medical devices.
Key Responsibilities:
- Develop research protocols and study designs.
- Conduct in-depth analysis of trial data.
- Write scientific reports and publications.
- Collaborate with research teams and regulatory bodies.
- Innovate new approaches for clinical studies.
Skills Required:
- Strong research and analytical skills.
- Ability to interpret complex scientific data.
- Scientific writing and publication expertise.
- Collaboration with cross-functional teams.
The field of clinical research offers numerous career opportunities for graduates in the life science field, such as biotechnology and related fields. Whether you are interested in data management, regulatory affairs, project management, or scientific research, there are many roles that will suit your skills and area of interest.
If you are passionate about medical innovation and improving patient care, pursuing a career in clinical research can be highly rewarding. Start building your skills and exploring opportunities today!
Ready to Launch Your Clinical Research Career?
Clinical Research Training 2025
Starting May 5 | 100% Online
Choose from 1, 3, or 6-month project durations!
With Placement Support to Kickstart Your Career
Apply Now: https://btnk.org/clinical-research-training
Speak with an Expert: https://btnk.org/contact-CR-Expert