Regulatory Affairs vs. Clinical Research vs. Pharmacovigilance – What’s Best For You?
Want to break into the booming pharmaceutical and biotech industry but are confused about where to start? You’re not alone!
With numerous career options like Regulatory Affairs, Clinical Research, and Pharmacovigilance, how do you choose the right path?
- Do you love analyzing data and running clinical trials?
- Are you detail-oriented and interested in drug approvals?
- Or do you want to ensure medicines are safe for millions of people?
Each field plays a critical role in drug development, but their job roles, skills, and career growth opportunities differ. Choosing the wrong path could mean spending years in a career that doesn’t excite you!
Before you decide, read this detailed breakdown of all three fields—so you can pick the perfect career and get ahead in biotech and pharma.
Table of Contents
Regulatory Affairs
The field of Regulatory Affairs (RA) ensures pharmaceutical and biotech products meet all necessary legal, ethical, and scientific standards required globally.
RA professionals collaborate closely with government agencies, research teams, and manufacturers to ensure
compliance throughout drug development.Role in Drug Development:
- Involved from early research to post-marketing surveillance
- Navigate regulatory approval processes
- Ensure compliance with industry standards
Key Responsibilities:
- Regulatory strategy development
- Regulatory submissions (IND, NDA, BLA, MAA)
- Compliance with international regulations (FDA, EMA, MHRA, DCGI, PMDA, WHO)
- Labeling and advertising compliance
- Post-approval monitoring
- Liaising with regulatory agencies
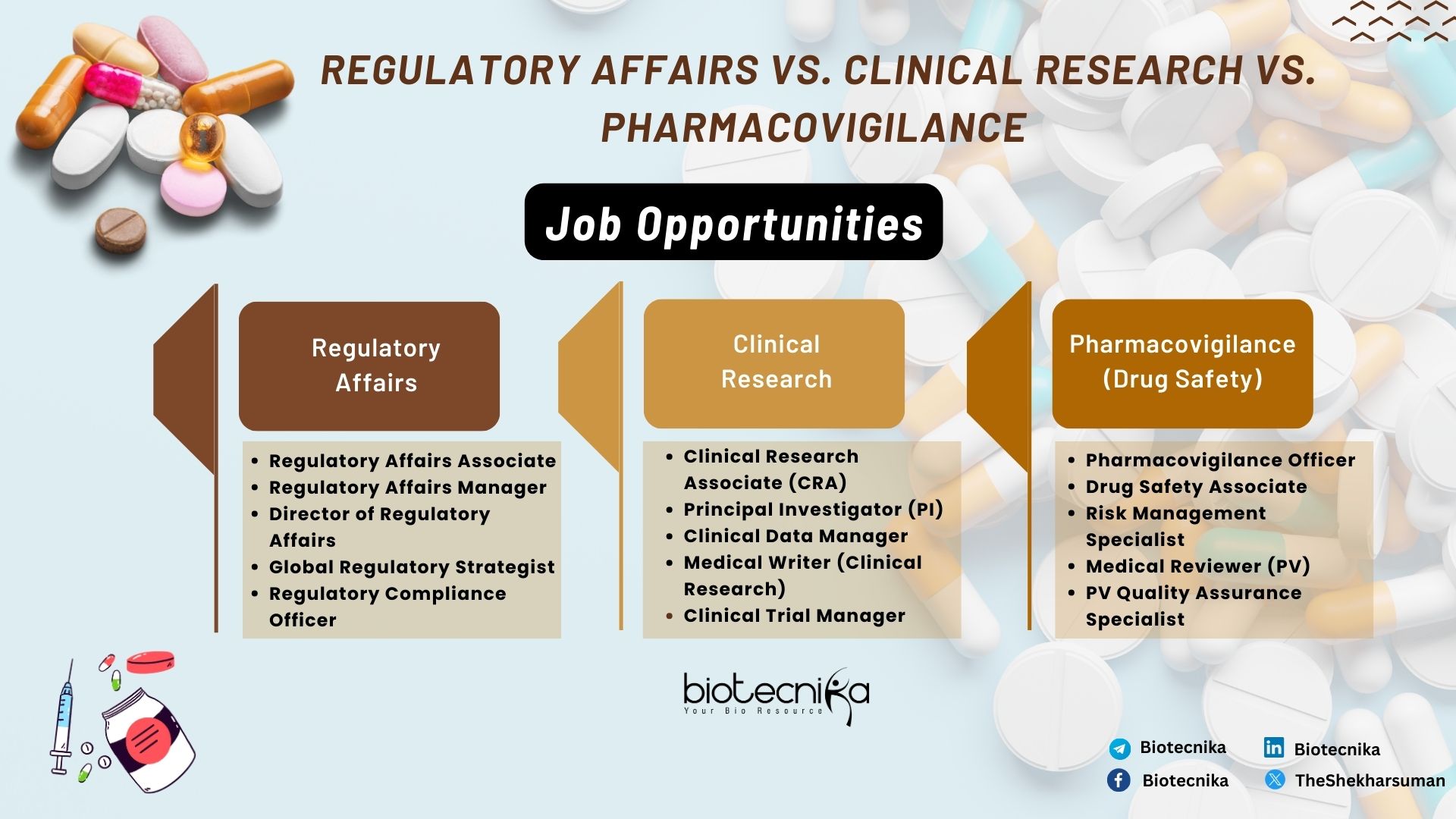
Job Opportunities:
- Regulatory Affairs Associate
- Regulatory Affairs Manager
- Director of Regulatory Affairs
- Global Regulatory Strategist
- Regulatory Compliance Officer
Skills Needed:
- Understanding GMP, GCP, and regulatory laws
- Technical writing and documentation
- Interpreting and applying regulations
- Attention to detail
- Communication and negotiation skills
Interested in Regulatory Affairs? Check out this Global Regulatory Affairs Hands-On Training Program with Live Project Work.
Clinical Research
Clinical Research is crucial for drug development, involving clinical trials to test new drugs, biologics, and medical devices for safety and efficacy.
Role in Drug Development:
- Manage clinical trials (Phases I-IV)
- Collect and analyze patient data
- Ensure patient safety and compliance
Key Responsibilities:
- Protocol development and study design
- Patient recruitment and informed consent
- Clinical trial management
- Statistical analysis and data collection
- Compliance with ICH-GCP guidelines
- Clinical trial documentation (IBs, SAE reports, ICFs)
Job Opportunities:
- Clinical Research Associate (CRA)
- Principal Investigator (PI)
- Clinical Data Manager
- Medical Writer (Clinical Research)
- Clinical Trial Manager
Skills Needed:
- Clinical trial phases, regulatory requirements, and ICH-GCP knowledge
- Statistical software proficiency
- Cross-functional teamwork
- Handling large datasets and attention to detail
Pharmacovigilance (Drug Safety)
Pharmacovigilance (PV) focuses on detecting, monitoring, and preventing adverse drug reactions, ensuring ongoing drug safety after approval.
Role in Drug Development:
- Post-marketing safety monitoring
- Real-world patient data analysis
Key Responsibilities:
- Adverse event reporting (ADRs, SAEs, UADRs)
- Signal detection and regulatory actions
- Risk management strategies (REMS)
- Post-marketing surveillance
- Regulatory reporting and compliance (PSURs)
Job Opportunities:
- Pharmacovigilance Officer
- Drug Safety Associate
- Risk Management Specialist
- Medical Reviewer (PV)
- PV Quality Assurance Specialist
Skills Needed:
- ICH E2E guidelines, regulatory reporting, MedDRA coding
- Critical thinking and analytical skills
- Medical case report evaluation
- Attention to detail and knowledge of drug interactions
Career Path & Key Differences Comparison: Regulatory Affairs vs Clinical Research vs Pharmacovigilance
| Key Aspects | Regulatory Affairs | Clinical Research | Pharmacovigilance |
|---|---|---|---|
| Primary Focus | Regulatory compliance and approvals | Conducting clinical trials | Monitoring drug safety |
| Role in Drug Lifecycle | Pre-approval and post-marketing compliance | Clinical trials (Phase I-IV) | Post-marketing surveillance and adverse event reporting |
| Regulatory Framework | FDA, EMA, ICH, CDSCO | GCP, ICH Guidelines | WHO, FDA, CIOMS, EMA |
| Key Deliverables | Regulatory dossiers, compliance reports | Clinical trial reports, patient data | Safety reports, risk-benefit analyses |
| Work Environment | Pharma companies, regulatory bodies | Hospitals, CROs, research institutes | Pharma companies, Regulatory agencies |
| Required Background | Life sciences, regulatory sciences | Life sciences, clinical research | Pharmacy, Pharmacology, life sciences |
Which Career Path is Right for You?
- Choose Regulatory Affairs if regulatory compliance documentation and global agency interactions appeal to you.
- Opt for Clinical Research if clinical trials, medical research, and patient interaction excite you.
- Consider Pharmacovigilance if you are interested in drug safety, real-world impacts, and adverse event monitoring.
Each field is essential to drug development, ensuring medicine safety, compliance, and efficacy from development to market surveillance. Evaluate your interests, career goals, and strengths carefully. Each domain offers promising and fulfilling opportunities in life sciences.