Machine Learning in Drug Discovery: Jobs, Skills, and Trends
Welcome to the new era of healthcare and medicine, where AI (Artificial Intelligence) and ML (Machine Learning) converge with drug discovery to reform how we identify, develop, and deliver therapies. Imagine a future where diseases and disorders are diagnosed and treated with great precision and speed; personalized medicines are customized for each individual. The potential of AI & ML in drug discovery and the healthcare sector is immense—to unlock the secrets of human health with the help of powerful algorithms and tools.
But before we delve into the depths of this exciting and emerging field, let’s take a moment to appreciate the challenge it seeks to overcome. Conventional drug discovery is like searching for microscopic keys in a vast and intricate biological library. This key unlocks a specific door—a disease-causing mechanism—within the even more complex labyrinth of the human body. It’s an expensive, laborious, and time-consuming process, frequently taking billions of dollars and decades to launch a novel drug in the global market. However, ML offers a new approach, acting like a guide and a powerful ally to illuminate the way and dramatically accelerate the development of life-saving therapies.
The article “Machine Learning in Drug Discovery: Jobs, Skills, and Trends” explores the essential skills, job opportunities, and emerging bioinformatics trends shaping the world.
Table of Contents
Why is Drug Discovery Challenging?
Imagine searching for a microscopic key hidden in a massive and intricate library. This key unlocks the particular door—a disease-causing mechanism—within the even more complex labyrinth of the human body. That’s the challenge of traditional drug discovery: a laborious, expensive, and time-consuming process, often taking over a decade and billions of dollars to bring a single new drug to market.
The drug discovery journey involves identifying promising drug targets, finding molecules that can effectively interact with them (keys that fit the lock), and navigating the complexities of clinical trials to ensure safety and efficacy. It’s a process fraught with challenges, including:
-
The vastness of biological data: Researchers must sift through mountains of genomic, proteomic, and clinical information to identify relevant targets and understand disease mechanisms.
-
The complexity of molecular interactions: Predicting how a molecule will interact with a biological target requires sophisticated computational models and experimental validation.
-
The high cost and long duration of clinical trials: Bringing a novel drug to market requires extensive clinical testing, which can be prone to failure, expensive, and time-consuming.
Machine Learning: A Powerful Toolkit for Drug Discovery
ML offers a powerful searchlight and sophisticated tools to navigate this complex maze. By analyzing vast datasets and identifying patterns invisible to the naked eye, ML is transforming drug discovery in several key ways:
-
Accelerating Target Identification: ML algorithms can analyze vast datasets of genomic, proteomic, and clinical information to pinpoint promising drug targets. This involves identifying genes, proteins, or pathways that play a critical role in diseases. For instance, Random Forests, a type of ensemble learning method, can be used to rank potential targets based on their predicted relevance to the disease.
-
Optimizing Lead Discovery: Once a target is identified, researchers need to find a molecule that can effectively interact with it. ML can predict the properties of molecules, enabling researchers to design and screen potential drug compounds more efficiently. Techniques such as QSAR (Quantitative Structure-Activity Relationship) modeling and deep learning help researchers screen thousands of compounds in silico
, which means the process is conducted on a computer, narrowing down candidates for laboratory testing. -
Predicting Clinical Trial Outcomes: Clinical trials are a critical but expensive and time-consuming step in drug development. ML models can analyze patient data to identify individuals most likely to benefit from a new therapy, improving clinical trial design and success rates. This can be achieved using logistic regression or more complex deep learning models.
-
Drug Repurposing: By analyzing existing data, ML identifies new therapeutic uses for approved drugs, shortening development timelines significantly. This involves analyzing diverse data sources, like clinical trial results and scientific literature, to uncover hidden connections between diseases and drugs. For instance, a drug initially developed to treat one condition may show promise in treating a completely different disease due to shared biological pathways or mechanisms.
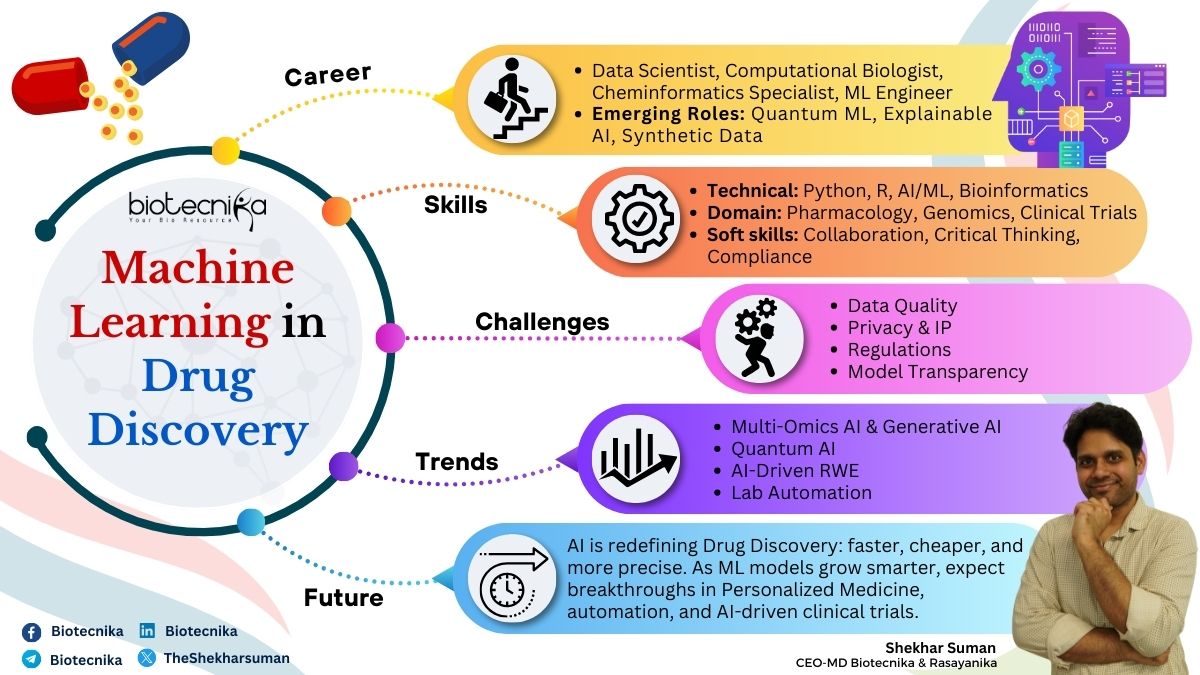
The Evolving Landscape: Skills & Career in ML-Driven Drug Discovery
The integration of ML in drug discovery creates diverse career opportunities at the intersection of life sciences and technology. These roles span a range of disciplines, catering to both technical experts and those with domain-specific knowledge:
-
Data Scientist: Specializing in analyzing and interpreting complex biological data, these professionals develop ML models to support drug discovery efforts. They work with datasets from proteomics, clinical trials, and genomics, requiring statistics, domain knowledge proficiency, and programming. Data scientists can specialize in various areas, such as proteomics, clinical data analysis, or genomics. Career progression often involves moving from junior to senior roles and potentially leading data science teams.
-
Computational Biologist/Bioinformatician: Experts in biological data analysis use ML to decode ’omics datasets—large-scale datasets that provide comprehensive information about a specific biological system—and simulate biological processes. Their work often bridges the gap between raw biological data and actionable insights for drug development. Bioinformaticians can specialize in areas like sequence analysis, structural bioinformatics, or systems biology. Career paths often lead to senior scientist roles or leadership positions in bioinformatics teams.
-
Cheminformatics Specialist: With expertise in chemistry and ML, these professionals design and implement algorithms for virtual screening, molecular docking, and predictive modeling of chemical interactions. They play a crucial role in lead compound identification. Cheminformaticians can specialize in areas like molecular modeling, virtual screening, or QSAR modeling. They can advance to senior scientist roles or lead cheminformatics teams.
-
AI Research Scientist: Focused on advancing ML techniques, these scientists create novel algorithms tailored to drug discovery challenges. Their work often involves developing generative models, reinforcement learning frameworks, and explainable AI tools. AI research scientists usually have PhDs in computer science or related fields and may progress to senior research positions or leadership roles in AI research teams.
-
Machine Learning Engineer: These are the architects of the ML algorithms used in drug discovery. They design, develop, implement, and optimize models for various applications, such as target identification, lead optimization, and clinical trial prediction. They need a strong understanding of different ML techniques, including deep learning, and proficiency in programming languages like Python. A typical career path might start with a role as a junior ML engineer, progressing to senior ML engineer, lead ML engineer, and potentially even director of AI.
Essential Skills for a career in AI and ML
Professionals aiming to thrive in ML-driven drug discovery must cultivate a blend of domain knowledge, technical skills, and soft skills:
-
Technical Skills: Proficiency in programming languages (Python, R), data analysis and visualization tools, ML frameworks (TensorFlow, PyTorch), molecular modeling software, statistical analysis, and bioinformatics tools.
-
Domain Knowledge: Understanding of pharmacology, genomics, proteomics, clinical research, medicinal chemistry, and systems biology.
-
Soft Skills: Interdisciplinary collaboration, problem-solving, communication, adaptability, project management, and critical thinking.
Opportunity To Get Hands-on Experience in ML-Driven Biology & Bioinformatics!
If you’re serious about building a career at the intersection of machine learning and drug 
Biotecnika is offering an exclusive Summer Hands-on Training Program on AI and Machine Learning in Biology & Bioinformatics, tailored for aspiring professionals, researchers, and students like you.
What Makes This Training Unique?
-
Work on Real-Time Research Projects: Gain practical experience in applying ML algorithms to biological datasets.
-
Get Published: Select participants will get the opportunity to co-author scientific research papers based on their project work.
-
Learn Tools That Matter: From Python and R programming to bioinformatics databases and ML frameworks, the course covers all the essential tools needed in modern drug discovery and computational biology.
-
Work Experience Certificate: Receive a certificate that boosts your academic and professional profile, proving your skills to future employers and research institutions.
Limited Seats Available – Enroll Now:
Join the Summer Hands-on Training Program
WORK IN PROJECTS + PUBLISH PAPERS __________________________________________
Whether you want to enter the biotech industry, pursue a PhD, or publish research, this program helps you build the skills and portfolio needed to succeed in ML-powered drug discovery.
Shaping the Future: Trends in ML-Driven Drug Discovery
The field is rapidly evolving, with several trends shaping its trajectory:
-
Integration of multi-omics data: Combining genomics, proteomics, metabolomics, and transcriptomics datasets allows for a holistic understanding of diseases and drug responses.
-
Generative models for drug design: GANs (Generative Adversarial Networks) and VAEs (Variational Autoencoders) are being used to create novel molecular structures with desired properties.
-
Quantum computing: Quantum algorithms hold the promise of revolutionizing molecular simulations, enabling more accurate predictions of molecular interactions.
-
Real-world evidence (RWE) utilization: ML analyzes RWE from EHRs (Electronic Health Records) and wearable devices to guide drug development.
-
Automation and robotics: Robotic systems integrated with ML algorithms automate high-throughput screening and laboratory experiments.
-
Explainable AI (XAI): XAI techniques are becoming essential to ensure that ML models’ decisions are interpretable and trustworthy.
The Challenges
While ML offers immense potential, it also comes with challenges:
-
Data Quality and Accessibility: Inconsistent or incomplete datasets can hinder model accuracy.
-
Bias in Algorithms: It is critical to ensure that ML models are free from biases that could lead to inequitable healthcare outcomes.
-
Intellectual Property and Data Privacy: Balancing innovation with patient confidentiality and proprietary rights remains challenging.
-
Regulatory Compliance: Navigating the evolving regulatory framework for AI-driven tools requires constant vigilance.
Despite these challenges, the future of ML in drug discovery is bright. As computational power grows and datasets expand, ML models will become increasingly sophisticated, leading to faster, safer, and more effective therapies. The demand for skilled professionals in this domain will continue to rise, offering exciting opportunities for those at the intersection of biology, chemistry, and data science. By staying abreast of trends, acquiring relevant skills, and embracing interdisciplinary collaboration, individuals can contribute to this transformative era in drug discovery.
In conclusion, ML is not merely a tool but a catalyst for redefining the future of drug discovery. As the industry evolves, so must its workforce, which must embrace change and innovation to unlock new possibilities in the healthcare sector.