Benefits of Knowing Regulatory Affairs
The Unsung Hero of Life Sciences
Imagine this: A revolutionary cancer drug has been discovered. Scientists and researchers rejoice. Patients see it as a ray of hope. The media calls it a ‘miracle cure.’ But months pass, then years. The drug never makes it to the market. Why? Regulatory hurdles.
In the world of biotech and pharmaceuticals, brilliant science alone is not enough. Without regulatory approval, even the most groundbreaking and effective drugs remain trapped in laboratories. This is why every professional in the industry must be aware of Regulatory Affairs (RA). Let’s explore why this knowledge is not just beneficial, but essential.
Table of Contents
Deciphering Regulatory Affairs
No Regulatory Approval, No Market Entry
Did you know? Nearly 90% of drug candidates fail clinical trials, with many failures resulting not from poor science but from regulatory misalignment or inadequate trial design. A drug’s journey from its discovery in the lab to market can take over 10 years or more and cost up to $2.6 billion on average.
Understanding regulatory requirements early can:
- Accelerate product development
- Mitigate costly trial failures
- Inherent compliance from the very first day
Failing to align with regulatory expectations at any step of the process can result in:
- Protracted approval timelines – In case submissions fail to adhere to regulations, they will be subjected to supplementary studies and re-evaluation. Pushing timelines back by years.
- Trial failures – Inadequately designed clinical trials are a setup for failure. Failing to align with prescribed regulatory endpoints might raise regulatory and ethical dilemmas.
- Market Retraction– Even after the drug/ therapeutic is initially approved, if post-market regulatory issues arise, the drug will be withdrawn from the market immediately. Leading to various lawsuits being filed and damage to corporate reputation.
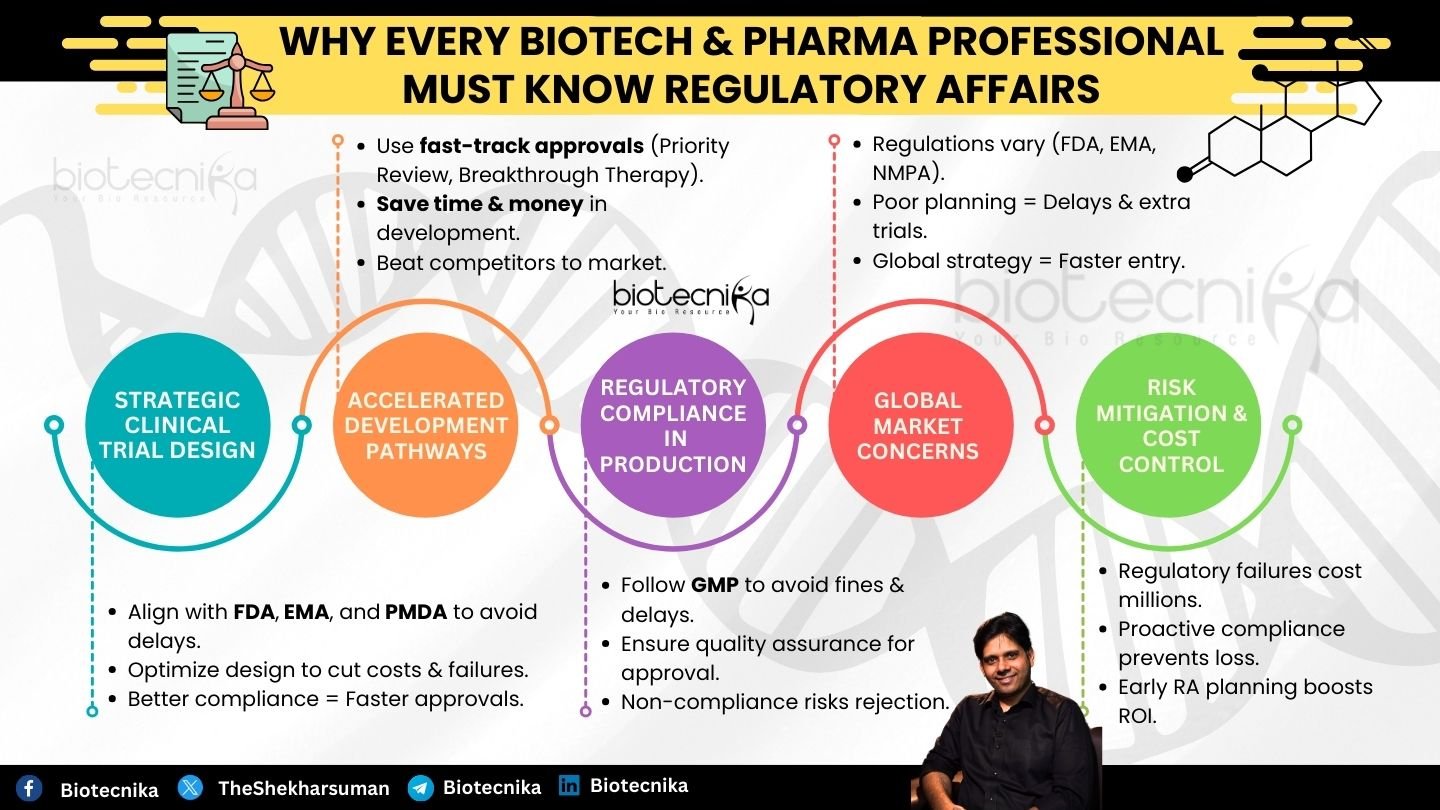
Why Understanding Regulatory Affairs Early is Critical?
- Strategic Clinical Trial Design
- A significant proportion of trial failures occurs due to the misalignment of clinical trial protocols with regulatory prescriptions.
- Regulatory bodies such as FDA, EMA, and PMDA have stringent guidelines regarding safety, clinical endpoints, trial populations, and the statistical methods used. Not being aware of this critical information could lead to a waste of resources.
- Companies that have regulatory expertise can design better trials, reducing trial failure.
- Accelerated Development Pathways
- Regulatory agencies have expertise in these areas. Hence, they can fastrack the process. Accelerated Approval and Priority Review to expedite drug development for critical unmet medical needs.
- However, navigating these pathways requires in-depth regulatory knowledge. Entities that fail to capitalize on these opportunities risk spending unnecessary years and money in development, while competitors possessing regulatory acumen bring products to market faster.
- Regulatory Compliance in Manufacturing
- Even in instances where the drug demonstrates efficacy, failure to meet Good Manufacturing Practices (GMP) can prevent market approval.
- Regulatory authorities conducted meticulous examinations of manufacturing facilities; any deviation from SOPs can results in warning letters, penalties, and delays in market release.
- Professionals understand quality assurance, process validation, and regulatory documentation can prevent these costly setbacks.
- Global Market Concerns
- Regulatory requirements exhibit heterogeneity across the international landscape, due to which there might be complications and re-evaluations if required.
- Understanding the divergences between FDA, EMA, and other regulatory bodies allows companies to develop a global regulatory strategy, thereby minimizing the need for redundant trials and facilitating expediting worldwide market entry.
- Risk Mitigation and Cost Control
- Drug development is a tedious procedure involving hundreds of millions of dollars; regulatory transgressions can impose financial burdens upon failure.
- Proactive investment in regulatory compliance audits and expert consultations can mitigate this kind of financial loss.
- Enterprises that have regulatory planning integrated into their R&D and business strategies are in a better position to avert financial failures and maximize their ROI.
Global Expansion Needs Regulatory Awareness
The Biotech and Pharmaceutical ecosystem operates inherently on a global scale, but the regulatory guidelines have significant divergence across different parts of the globe. Enterprises must skillfully navigate through the regulatory matrix to soft launch their products to the market.
Key Regulatory Challenges Across Significant Markets
- United States (FDA) – The Food and Drug Administration mandates rigorous clinical trials consisting of meticulous safety and efficacy data and an unwavering alignment with Good Clinical Practices (GCP).
- Europe (EMA) – Focuses on a benefit-risk assessment model, requiring comprehensive data and public transparency through European Public Assessment Reports (EPARs).
- India (CDSCO) – Fast-tracking approvals for critical drugs, allowing waivers for local trials if the drug is approved in key global markets.
- China (NMPA) – Adapting international standards but still requiring local pharmacokinetic (PK) studies and strict post-market surveillance.
- Japan (PMDA) – Demands country-specific clinical trials and extensive risk management plans (RMPs) for post-market safety.
Navigating Clinical Trials & Compliance
Clinical trials are just about scientific discovery and innovation; they must simultaneously adhere to stringent regulatory and ethical guidelines prescribed. This duality ensures patient safety, data protection, and integrity, eventually leading to the attainment of regulatory approval.
Key Compliance Requirements in Clinical Trials
- ICH-GCP (International Council for Harmonisation –Good Clinical Practices) – Internationally recognized standards ensuring ethical and scientifically sound study design, conduct, and reporting.
- Ethical Guidelines for Human Subjects – Adherence to the Declaration of Helsinki, Institutional review board (IRB) approvals, and informed consent processes to safeguard patient rights.
- Data Integrity & Transparency Laws – Regulations such as FDA 21 CFR Part 11 and EU Clinical Trial Regulation (CTR) mandate secure handling of trial data, precluding manipulation, fraud, or bias.

A Competitive Edge for Career Growth
In 2025, companies are placing greater emphasis on regulatory expertise, making it a valuable asset for career advancement. Professionals endowed with regulatory affairs (RA) knowledge earn 20-30% higher salaries and progress faster into leadership roles compared to their peers without regulatory experience.
Why Regulatory Knowledge Matters for Career Growth
- Bridges Science and Commercial Domains – RA professionals serve as pivotal intermediates between R&D, clinical trials, and market access, rendering them indispensable in strategic decision-making.
- High Demand, Limited Supply – Amidst the ever-evolving global regulations, companies are actively seeking professionals with regulatory expertise to ensure compliance and streamline product approvals.
- Diverse Career Opportunities – RA knowledge unlocks a plethora of opportunities across drug development, medical devices, biologics, and quality assurance, thereby providing job security.
Common Career Progression Paths
- R&D Scientist → Regulatory Consultant – Scientists with regulatory expertise transition into roles advising companies on compliance strategies.
- Clinical Trial Manager → RA Manager – Clinical professionals move into regulatory roles by overseeing trial submissions and approvals.
- Quality Assurance Expert → Regulatory Compliance Officer – Ensuring adherence to GMP, GCP, and global quality standards leads to administration positions in regulatory compliance.
Top Biotech & Pharma Companies Hiring RA Professionals:
- Global: Pfizer, Johnson & Johnson, AstraZeneca, Roche, Novartis
- India: Sun Pharma, Dr. Reddy’s, Cipla, Biocon, Serum Institute
Professional Associations & Certifications:
- Regulatory Affairs Professionals Society (RAPS)
- Postgraduate Diploma in Regulatory Affairs (Offered in India & Abroad)
As the African proverb goes, “Knowledge is like a garden: if it is not cultivated, it cannot be harvested.” In the biotech and pharma realm, regulatory knowledge is the garden that ensures scientific innovation bears fruit.
If you’re in this industry, understanding Regulatory Affairs is not an option—it’s your key to success. Whether the aspiration is to develop therapies or drugs, navigate clinical trials, or lead a company to global expansion, Regulatory knowledge will set you apart.
Conclusion & Your Next Step:
As biotechnology and pharmaceutical sectors continue to grow, the role of Regulatory 
To stay ahead and gain in-depth, practical knowledge of Regulatory Affairs, consider enrolling in our Global Regulatory Affairs Hands-on Training Program with Live Project Work. This training program is designed to equip you with real-world skills, industry-recognized expertise, and valuable project experience, making you indispensable to any biotech or pharma organization.
Don’t wait—regulatory knowledge today means career excellence tomorrow.
Benefits of Knowing Regulatory Affairs


































