Breaking News: Oxygen’s Shocking Transformation Will Leave You Breathless
In a groundbreaking discovery, scientists have stumbled upon a brand-new variant of oxygen that defies conventional expectations. This isotope, known as oxygen-28, holds the record for the highest number of neutrons ever witnessed within an oxygen atom’s nucleus. Astonishingly, despite predictions of stability, it undergoes rapid decay, challenging our understanding of fundamental particle arrangements within an atom’s nucleus.
Oxygen’s Shocking Transformation: Oxygen-28 has captured attention due to its intriguing behaviour. Unlike its stable counterpart, oxygen-16, which contains 8 protons and 8 neutrons, oxygen-28 boasts an unusually high count of neutrons. This brings into focus the concept of isotopes, where elements share the same number of protons but possess varying numbers of neutrons. The team of researchers, led by nuclear physicist Yosuke Kondo of the Tokyo Institute of Technology, embarked on a novel experiment at the RIKEN Radioactive Isotope Beam Factory to investigate this unusual oxygen variety.
The researchers began their inquiry by firing a beam of calcium-48 isotopes at a beryllium target, generating lighter atoms such as fluorine-29—a fluorine isotope with 9 protons and 20 neutrons.
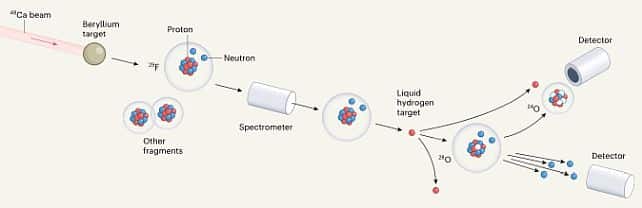
The goal was to generate oxygen-28 through a collision
involving fluorine-29 and a liquid hydrogen target, where a proton would be detached, leading to the formation of oxygen-28. Although successful, the outcome was not expected. Both oxygen-27 and oxygen-28 exhibited instability, swiftly decaying into oxygen-24 along with 3 or 4 loose neutrons.These are specific counts of protons or neutrons that indicate a stable configuration, akin to a filled energy shell. Oxygen-28’s neutron count of 20 should theoretically render it stable due to this magic number, yet it defied this expectation.
The discovery challenges our understanding of the neutron shell and its connection to stability. Previous assumptions about magic numbers and their influence on an isotope’s stability are now in question. Oxygen-28’s behaviour seems consistent with a phenomenon termed the “island of inversion,” observed in isotopes of other elements like neon, sodium, and magnesium, where the expected stability does not align with reality.
Despite this revelation, there remain unresolved questions about the neutron shell and its peculiar characteristics. Deeper insights into this enigma await further studies conducted on the nucleus in a heightened energy state. Additionally, alternative methods of oxygen-28 formation could provide crucial clues, although this avenue presents significant challenges.
In conclusion, the discovery of oxygen-28’s unexpected behaviour has shed light on the complexity of doubly magic nuclei. This revelation not only challenges our existing knowledge of particle arrangements but also underscores the intricate nature of fundamental particles within the atom. As the scientific community delves further into this uncharted territory, the tantalizing secrets of oxygen-28 continue to unfold, reshaping our understanding of the atomic world.
EXPLORE BIOTECNIKA FOR MORE UPDATES



























